BONLAB BLOG
Thoughts
&
Scientific Fiction
Roughening up polymer microspheres and their diffusion in a liquid
Spherical microparticles that are roughened up, so that their surfaces are no longer smooth, are intriguing. You can wonder that when we place a large number of these particles in a liquid, it may show interesting rheological behaviour. For example, would they behave like cornstarch in that when we apply a lot of shear it thickens? You can imagine that spiky spheres can interlock and jam. Biologists are interested in how microparticles interact with cells and organisms, and have started to show that the shape of the particle can play an important role. Similarly, these small particles of intricate shape may show fascinating behavior at deformable surfaces, for example is there a cheerio effect?, and may show unexpected motion. This sounds all fun, but how do we make rough microparticles, as for polymer ones this is not easy?
Spherical microparticles that are roughened up, so that their surfaces are no longer smooth, are intriguing. You can wonder that when we place a large number of these particles in a liquid, it may show interesting rheological behaviour. For example, would they behave like cornstarch in that when we apply a lot of shear it thickens? You can imagine that spiky spheres can interlock and jam. Biologists are interested in how microparticles interact with cells and organisms, and have started to show that the shape of the particle can play an important role. Similarly, these small particles of intricate shape may show fascinating behavior at deformable surfaces, for example is there a cheerio effect?, and may show unexpected motion. This sounds all fun, but how do we make rough microparticles, as for polymer ones this is not easy?
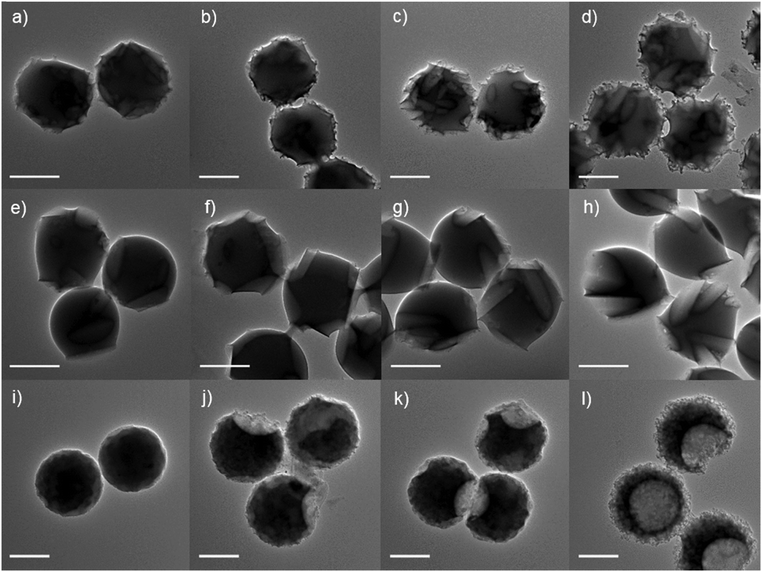
Fig. 1 Transmission electron microscopy (TEM) images of poly(styrene) microspheres deformed at 110 °C within a dried colloidal inorganic matrix for approximate time periods of 10, 30, 60 and 120 min (a–d), (e–h), (i–l). The inorganic particles utilized were cigar-shaped calcium carbonate (a–d), large, rod-shaped calcium carbonate (e–h) and small, spherical/oblong-shaped zinc oxide (i–l). Scale bars = 1.0 μm.
In our paper published in Soft Matter we report an easy and versatile method to morph spherical microparticles into their rough surface textured analogues. For this, we embed the particles into an inorganic matrix of intricate shape and heat them up above the temperature at which the particles become a polymer melt. Capillary imbibition imprints the inorganic texture into the particles, turning them rough. We also look at how the particles move through a liquid, by tracking their motional behavior. Rough particles appear bigger, than their smooth precursors.
You can read the paper here: DOI:10.1039/C7SM00589J
A mechanistic investigation of Pickering emulsion polymerization
Emulsion polymerization is an important industrial production method to prepare latexes. Polymer latex particles are typically 40-1000 nm and dispersed in water. The polymer dispersions find application in wide ranges of products, such as coatings and adhesives, gloves and condoms, paper textiles and carpets, concrete reinforcement, and so on.
Conventional emulsion polymerization processes make use of molecular surfactants, which aids the polymerization reaction during which the particles are made and keeps the polymer colloids dispersed in water. We, and others, introduced Pickering emulsion polymerization a decade ago in which we replace common surfactants with inorganic nanoparticles.
In Pickering emulsion polymerization the polymer particles made are covered with an armor of the inorganic nanoparticles. This offers a nanocomposite colloid which may have intriguing properties and features not present in conventional "naked" polymer latexes.
To fully exploit this innovation in emulsion polymers, a mechanistic understanding of the polymerization process is essential. Current understanding is limited which restricts the use of the technique in the fabrication of more complex, multilayered colloids.
Emulsion polymerization is an important industrial production method to prepare latexes. Polymer latex particles are typically 40-1000 nm and dispersed in water. The polymer dispersions find application in wide ranges of products, such as coatings and adhesives, gloves and condoms, paper textiles and carpets, concrete reinforcement, and so on.
Conventional emulsion polymerization processes make use of molecular surfactants, which aids the polymerization reaction during which the particles are made and keeps the polymer colloids dispersed in water. We, and others, introduced Pickering emulsion polymerization a decade ago in which we replace common surfactants with inorganic nanoparticles.
In Pickering emulsion polymerization the polymer particles made are covered with an armor of the inorganic nanoparticles. This offers a nanocomposite colloid which may have intriguing properties and features not present in conventional "naked" polymer latexes.
To fully exploit this innovation in emulsion polymers, a mechanistic understanding of the polymerization process is essential. Current understanding is limited which restricts the use of the technique in the fabrication of more complex, multilayered colloids.
In our paper, recently published in Polymer Chemistry, clarity is provided through an in-depth investigation into the Pickering emulsion polymerization of methyl methacrylate (MMA) in the presence of nano-sized colloidal silica (Ludox TM-40). Mechanistic insights are discussed by studying both the adsorption of the stabiliser to the surface of the latex particles and polymerization kinetics. The adhesion of the Pickering nanoparticles was found not to be spontaneous, as confirmed by cryo-TEM analysis of MMA droplets in water and monomer-swollen PMMA latexes. This supports the theory that the inorganic particles are driven towards the interface as a result of a heterocoagulation event in the water phase with a growing oligoradical. The emulsion polymerizations were monitored by reaction calorimetry in order to establish accurate values for monomer conversion and the overall rate of polymerizations (Rp). Rp increased for higher initial silica concentrations and the polymerizations were found to follow pseudo-bulk kinetics.
The paper can be read here: http://dx.doi.org/10.1039/C7PY00308K
Independent responsive behaviour and communication in hydrogel objects
Autonomous response mechanisms are vital to the survival of living organisms and play a key role in both biological function and independent behaviour. The design of artificial life, such as neural networks that model the human brain and robotic devices that can perform complex tasks, relies on programmed intelligence so that responses to stimuli are possible. Responsive synthetic materials can translate environmental stimuli into a direct material response, for example thermo-responsive shape change in polymer gels or light-triggered drug release from capsules. Materials that have the ability to moderate their own behaviour over time and selectively respond to their environment, however, display autonomy and more closely resemble those found in nature.
Autonomous response mechanisms are vital to the survival of living organisms and play a key role in both biological function and independent behaviour. The design of artificial life, such as neural networks that model the human brain and robotic devices that can perform complex tasks, relies on programmed intelligence so that responses to stimuli are possible. Responsive synthetic materials can translate environmental stimuli into a direct material response, for example thermo-responsive shape change in polymer gels or light-triggered drug release from capsules. Materials that have the ability to moderate their own behaviour over time and selectively respond to their environment, however, display autonomy and more closely resemble those found in nature.
In our recent paper, published in Materials Horizons, we present soft hydrogel objects that possess an individually programmed time delay in their response to a shared environmental stimulus. We utilize the enzyme urease to programme a self-regulated change in pH, which in turn activates the designed response of gel disintegration. This design allows for independent response behaviour of a collection of hydrogel fibers which contain coloured oil droplets in a single closed system. In addition, we show that hydrogel beads can communicate with one another, hereby influencing their pre-programmed individual behaviour.
The incorporation of responsive time control directly into soft matter objects demonstrates an advance in the field of autonomous materials.
The paper can be read at http://dx.doi.org/10.1039/C7MH00033B