BONLAB BLOG
Thoughts
&
Scientific Fiction
Innovation in Emulsion Polymerization process opens window to Janus and patchy particles
Emulsion polymerization is of pivotal importance as a route to the fabrication of water-based synthetic polymer colloids. The product is often referred to as a polymer latex and plays a crucial role in a wide variety of applications spanning coatings (protective/decorative/automotive), adhesives (pressure sensitive/laminating/construction), paper and inks, gloves and condoms, carpets, non-wovens, leather, asphalt paving, redispersible powders, and as plastic material modifiers.
Since its discovery in the 1920s the emulsion polymerization process and its mechanistic understanding has evolved. Our most noticeable past contributions include the first reversible-deactivation nitroxide-mediated radical emulsion polymerization (Macromolecules 1997: DOI 10.1021/ma961003s), and the development and mechanistic understanding of Pickering mini-emulsion (Macromolecules 2005: DOI 10.1021/ma051070z) and emulsion polymerization processes (J. Am. Chem. Soc. 2008: DOI 10.1021/ja807242k). The latest on nano-silica stabilized Pickering Emulsion Polymerization from our lab can be found here.
One quest in emulsion polymerization technology that remains challenging and intriguing is control of the particle morphology. It is of importance as the architecture of the polymer colloid influences its behavioural properties when used in applications. We now report in ACS Nano an elegant innovation in the emulsion polymerization process which makes use of nanogels as stabilizers and allows us to fabricate Janus and patchy polymer colloids.
Emulsion polymerization is of pivotal importance as a route to the fabrication of water-based synthetic polymer colloids. The product is often referred to as a polymer latex and plays a crucial role in a wide variety of applications spanning coatings (protective/decorative/automotive), adhesives (pressure sensitive/laminating/construction), paper and inks, gloves and condoms, carpets, non-wovens, leather, asphalt paving, redispersible powders, and as plastic material modifiers.
Since its discovery in the 1920s the emulsion polymerization process and its mechanistic understanding has evolved. Our most noticeable past contributions include the first reversible-deactivation nitroxide-mediated radical emulsion polymerization (Macromolecules 1997: DOI 10.1021/ma961003s), and the development and mechanistic understanding of Pickering mini-emulsion (Macromolecules 2005: DOI 10.1021/ma051070z) and emulsion polymerization processes (J. Am. Chem. Soc. 2008: DOI 10.1021/ja807242k). The latest on nano-silica stabilized Pickering Emulsion Polymerization from our lab can be found here.
One quest in emulsion polymerization technology that remains challenging and intriguing is control of the particle morphology. It is of importance as the architecture of the polymer colloid influences its behavioural properties when used in applications. We now report in ACS Nano an elegant innovation in the emulsion polymerization process which makes use of nanogels as stabilizers and allows us to fabricate Janus and patchy polymer colloids.
False coloured SEM images of emulsion polymerizations using nanogels as stabilizers (N1) at 2.8 wt% wrt monomer in which the pH was adjusted to 8.8 (A), 5.5 (B), 5.0 (C) and 4.5 (D) prior to polymerization. Scale bars: 100 nm.
The use of the nanogels in the emulsion polymerization leads to anisotropic Janus and patchy colloids, where a latex particle is decorated with a number of patches on its surface. In the paper we show that control of particle size and patch density can be achieved by tailoring the reaction conditions.
Proposed mechanism for the formation of Janus and patchy particles in the emulsion polymerization of styrene carried out in presence of nanogel particles.
The work was carried out by a team of talented scientists from the BonLab, Andrea Lotierzo, Brooke Longbottom, and Wai Hin Lee. Prof.dr.ir. Stefan Bon says: “ I am absolutely delighted that our work is published in the internationally leading journal ACS Nano. It is a great achievement of the team who have worked tremendously hard in the realisation of this new innovative technology. It shows that the area of emulsion polymerization is very much alive and kicking!”
The link to the paper is here: DOI: 10.1021/acsnano.8b06557
BonLab joins the Bio Electricity Group and the Bio Electrical Engineering (BEE) Hub
The BonLab at Warwick University specialises in the fabrication of colloidal and macromolecular materials for a wide range of applications, including coatings/adhesives, personal/household care products, and confectionary. BonLab's recent scientific activity in the fields of autonomous and programmable colloidal gels and active colloidal matter drew attention from researchers in life sciences.
Prof.dr.ir. Stefan Bon says: "We are delighted with the invitation to join the bio electricity group and the bio electrical engineering (BEE) hub, hosted at Warwick University. We hope that our scientific portfolio and know-how will provide a synergistic angle and will help innovate in this exciting area of science"
Information on the Bio Electricity Group:
Despite the early works of Luigi Galvani in the 18th century, the experimental inquiry into the biological systems has never fully taken an electrical viewpoint. Galvani’s, and subsequently Alessandro Volta’s, studies led to the discovery of the electrical battery and the birth of electrochemistry, but the biological thread have been largely neglected outside of neurosciences.
At Warwick, we have taken on this neglected thread and have identified biological electricity as a key research direction. In particular, we believe that electrical forces, and the ability to control them, are fundamental in organising living systems across the scales (see publications). To better understand these forces and develop means to measure and control them, we undertake an interdisciplinary approach that brings together expertise from biology, physics, engineering, and chemistry.
Our research in this area is currently conducted through several collaborative PhD and postdoctoral projects. In addition, we have recently launched a Bio Electrical Engineering (BEE) Innovation Hub with funds from a BBSRC Innovation Accelarator Award provided to the University of Warwick.
Current membership (and interest areas) in the Warwick BioElectricity group include; Munehiro Asally (electrical patterns in cellular organisation), Orkun Soyer (electrical interfaces to cells), Murray Grant (electrical signals in plants), Pat Unwin (electrobiochemical measurements), Marco Polin (electrotaxis), Rob Cross (sub-cellular electrical fields), and Stefan Bon (electrical stimuli in colloidal biomaterials)
.
Assembly of colloidal latex particles leads to innovation in fabrication of porous materials
Porous materials that have an interconnected network of pores are an interesting class of materials and have drawn attention in the area of separation science. The ability to fabricate robust so-called open cellular materials with control of the porosity remains a scientific challenge. The ability of regulating the interconnected network determines how a fluid (liquid or gas) can flow through the system. Think for example of how water runs through soil, or how water can be taken up through capillary action into a sponge. In addition, one can foresee that matter which flows through the porous material can temporarily be adhered/adsorbed onto the surface of the porous monolithic structure. The ability to easily control the surface functionality of the walls of the pores therefore is important.
In collaborative work with Chris Desire, a talented PhD student from the group of prof. Emily Hilder at the University of South Australia, we in the BonLab describe in Green Chemistry that we can use polymer latex particles as colloidal building blocks to form robust open cellular porous monolithic materials by simply stacking them onto each other. This assembly process is triggered by colloidal instability of a polymer latex dispersed in water which leads to the formation of a colloidal gel. The structure of the gel can then be made permanent by cross-linking through polymerization.
Porous materials that have an interconnected network of pores are an interesting class of materials and have drawn attention in the area of separation science. The ability to fabricate robust so-called open cellular materials with control of the porosity remains a scientific challenge. The ability of regulating the interconnected network determines how a fluid (liquid or gas) can flow through the system. Think for example of how water runs through soil, or how water can be taken up through capillary action into a sponge. In addition, one can foresee that matter which flows through the porous material can temporarily be adhered/adsorbed onto the surface of the porous monolithic structure. The ability to easily control the surface functionality of the walls of the pores therefore is important.
In collaborative work with Chris Desire, a talented PhD student from the group of prof. Emily Hilder at the University of South Australia, we in the BonLab describe in Green Chemistry that we can use polymer latex particles as colloidal building blocks to form robust open cellular porous monolithic materials by simply stacking them onto each other. This assembly process is triggered by colloidal instability of a polymer latex dispersed in water which leads to the formation of a colloidal gel. The structure of the gel can then be made permanent by cross-linking through polymerization.
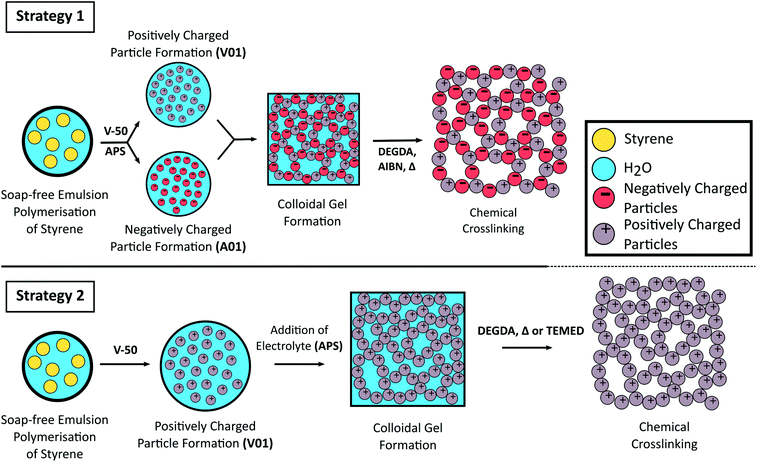
Schematic representation for the formation of crosslinked colloidal gels from oppositely charged latex particles prepared from the soap-free emulsion polymerisation of styrene using different initiators (Strategy 1) or from the addition of electrolyte to a cationic polymer latex (Strategy 2).
The pore size of the resulting monoliths was predictable as this was observed to directly correlate to the particle diameter, with larger pores achieved using particles of increased size. All gels obtained in this work were highly mouldable and retained their shape, which allowed for a range of formats to be easily prepared without the requirement of a mould.
Our innovation is applicable for the preparation of polymer monoliths for a wide variety of applications, including but not limited to, tissue engineering, catalysis, chromatography, extraction, sample preparation, and as absorbents. In particular these monoliths were found to posses relatively high porosities and were capable of rapidly absorbing solvents of varying polarity by capillary action, which suggested their applicability for thin layer chromatography (diagnostics) and extraction.
The original paper is published in Green Chemistry DOI:10.1039/C8GC01055B