BONLAB BLOG
Thoughts
&
Scientific Fiction
Grafting polymers to graphene oxide goes better through branching
Single-layer graphene is interesting as a flexible 2D material, with xy-dimensions variable up to a centimetre in length and a z-thickness of a single carbon atom. It conducts heat and electricity, has excellent mechanical strength, and is impermeable to gases except hydrogen gas. Its drawback: how to disperse it in a liquid. When you try to do this flexible sheets of graphene tend to stack as a result of attractive van der Waals interactions, making it virtually an impossible material to disperse as single sheets.
Single-layer graphene is interesting as a flexible 2D material, with xy-dimensions variable up to a centimetre in length and a z-thickness of a single carbon atom. It conducts heat and electricity, has excellent mechanical strength, and is impermeable to gases except hydrogen gas. Its drawback: how to disperse it in a liquid. When you try to do this flexible sheets of graphene tend to stack as a result of attractive van der Waals interactions, making it virtually an impossible material to disperse as single sheets.
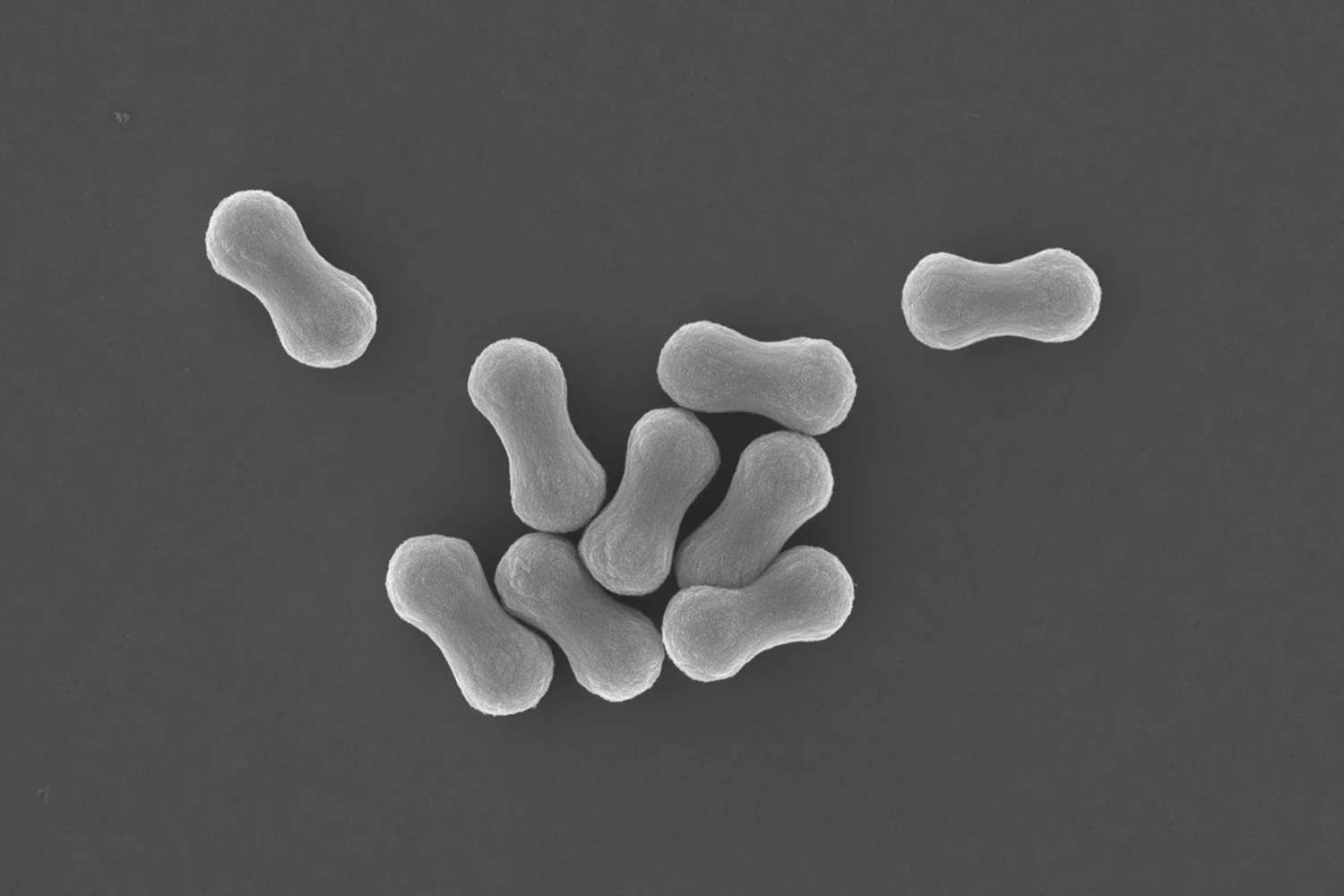
As a compromise, people came up with graphene oxide (GO), it’s oxidised analogue. The presence of oxygen atoms containing functional groups, such as hydroxy, epoxy, carboxylic acid, ketone, or aldehyde, provides it with a polarity, which makes it possible to disperse GO as single sheets in polar solvents, such as water or DMSO at low concentrations in the absence of electrolyte or other colloidal particles. The drawbacks: graphene oxide sheets have holes. Whereas graphene can be seen as a pristine, flexible bedsheet, graphene oxide is a bedsheet attacked by moths. This makes the material less suitable for barrier property reinforcement. Graphene oxide is also less electrically conducting as oxidation has partially destroyed its conjugation. Nevertheless, it still shows great potential for a range of applications, for example, those that need mechanical reinforcement, such as polymer nano-composites, but only if we can enhance its dispersibility in liquids or polymer melts.
Grafting polymer chains from the surface of GO allows it to be dispersed more easily. This can provide additional electrostatic and steric stabilization against sheet stacking at higher concentrations in less polar solvents or contain soluble polymer chains or non-interacting particles that can trigger depletion flocculation.
There are several approaches to accomplishing this, each with advantages and disadvantages. A particular challenge is linking a sufficient mass of polymer chains to the GO’s surface. In our gold open access paper recently published in the RSC journal Polymer Chemistry, we report a way to address this, employing radical polymerization reactions that lead to a branched polymer architecture. Methacrylate-based macromonomers made by catalytic chain transfer polymerization were used together as molecular weight regulators with dimethacrylates as crosslinkers to introduce branching points.
You can read the paper here:
BonLab designs a stick-on-demand adhesive for linerless labels
Labels are big business. A typical label has multiple layers: a topcoat for protection, the face stock, which contains the message in the form of text and/or images, a pressure-sensitive adhesive, and a release liner, which often has a release coating. The release liner and coating are only there to protect the label from sticking to things you do not wish it would stick to. You remove the liner when you wish to apply the label onto your substrate of choice, for example, a bottle containing a drink.
Imagine a label without a release liner and coating, imagine a label that could be activated at the moment you want it to stick to a substrate, a stick-on-demand linerless label.
BonLab has designed and developed a concept and prototype for a sustainable solution: a mesh reinforced pressure-sensitive adhesive for linerless label design.
Labels are big business. A typical label has multiple layers: a topcoat for protection, the face stock, which contains the message in the form of text and/or images, a pressure-sensitive adhesive, and a release liner, which often has a release coating. The release liner and coating are only there to protect the label from sticking to things you do not wish it would stick to. You remove the liner when you wish to apply the label onto your substrate of choice, for example, a bottle containing a drink.
Imagine a label without a release liner and coating, imagine a label that could be activated at the moment you want it to stick to a substrate, a stick-on-demand linerless label.
BonLab has designed and developed a concept and prototype for a sustainable solution: a mesh reinforced pressure-sensitive adhesive for linerless label design. The idea was worked out by Emily Brogden and prof. dr. ir. Stefan Bon, in collaboration with UPM Raflatac Oy, a global supplier of label materials for branding and promotion, information and functional labelling (patent application: WO2023105120A1). The complete study, which was done at the University of Warwick, is now published in the new journal RSC Applied Polymers.
A water-based pressure sensitive adhesive (PSA) is contained in a hard 3D mesh structure. This replaces the standard PSA layer of a label. It provides structural support upon storage and prevents the adhesive layer from sticking. In adhesive jargon: it shows excellent blocking resistance.
Upon short heat treatment, for example, upon contact with a hot sterilized glass bottle, the mesh will soften, allowing for adhesion to occur.
The 3D mesh structure was generated using a binary mixture of water-based polymer dispersions. One component is a standard PSA latex, and the other is a polystyrene polymer colloid. Film formation of the blend followed by a short annealing time led to the desired phase-separated mesh structure, as displayed in the figure below.
Visualization of the mesh structure in an acetone etched PVAc-PS model film (Film 1) a-c. a) SEM image of the top surface, b) a tilt adjusted image of the cross section after cutting away a section using FIB SEM with a film height of approximately 20 𝜇𝑚, and c) micro-CT 3D reconstruction of the etched film. Visualization of a similar mesh structure in a PSA-PS film (Film 2) d-f. d) SEM image of the top surface of Film 2, annealed for 60 𝑚𝑖𝑛 e) SEM image of the top surface (prepared similiarly to Film 2 but with only a 19 𝑚𝑖𝑛 annealing time) with a clean crack after freezing in liquid nitrogen and f) a cross section of Film 2, where the PS is tagged with hostasol methacrylate (green), taken using confocal microscopy (the original top surface of the cast film is at the top of the image).
Prof. dr. ir. Stefan Bon says: “I am immensely proud of the hard work done by all people involved to get the idea from a sketch on a piece of paper to a working prototype. A big thank you to PhD researcher Emily Brodgen, the paper's first author, who has been the driving force behind turning a concept into reality and has worked tremendously hard on the project for the last couple of years. The work has received excellent feedback from the academic and industrial scientific communities, with Emily winning multiple prizes for best talk and scientific poster.”
The paper can be accessed from the RSC Applied Polymers website:
https://doi.org/10.1039/D3LP00224A
A promotional video will be made available soon.
Triple awards for Emily, Josh, and Max.
A fantastic triple accomplishment by people from BonLab at the 2023 Chemistry graduation celebrations at the University of Warwick.
A fantastic triple accomplishment by people from BonLab at the 2023 Chemistry graduation celebrations at the University of Warwick.
From left to right. Emily Brogden, Josh Davies, Stefan Bon, and Max Campbell.
Emily Brodgen was the winner of a chemistry graduate teaching award, and Josh Davies was commended. These awards celebrate outstanding contributions to undergraduate laboratory teaching and demonstrating. Max Campbell won a chemistry students community building award, for his outstanding contributions to the Warwick Chemistry students’ community.
Prof. dr. ir. Stefan Bon says: “I am immensely proud of Emily, Josh, and Max. Not only do they do fantastic research in our team, they show that they also care and have tremendous talents and energy for the wider scientific community at Warwick. I am delighted their hard work and commitment has been recognized by the Chemistry Department in this years awards ceremony.”




