BONLAB BLOG
Thoughts
&
Scientific Fiction
Control of vesicle membrane permeability with catalytic particles
The ability to control membrane permeability in vesicles allows for regulated transport of matter across the vesicular wall. Vesicles can be seen as microscopic sacs containing a compartmentalized volume of liquid dispersed in a bulk liquid environment. Compartmentalization of small and finite volumes of liquid and consecutive the emergence of membrane bioenergetics are identified as being of key importance in the evolution of cells, and hence the origin of life. Nature has devised sophisticated strategies to accomplish control of transmembrane transport, including endo- and exocytosis as well as the incorporation of transmembrane proteins into cell membranes. A variety of synthetic approaches have been explored by scientists in order to accomplish such control in manmade systems. Examples include hybrid systems whereby transmembrane proteins were incorporated as part of synthetic vesicles, and the use of responsive macromolecular building blocks to regulate membrane porosity upon an external trigger in polymer vesicles, also referred to as polymersomes.
The ability to control membrane permeability in vesicles allows for regulated transport of matter across the vesicular wall. Vesicles can be seen as microscopic sacs containing a compartmentalized volume of liquid dispersed in a bulk liquid environment. Compartmentalization of small and finite volumes of liquid and consecutive the emergence of membrane bioenergetics are identified as being of key importance in the evolution of cells, and hence the origin of life. Nature has devised sophisticated strategies to accomplish control of transmembrane transport, including endo- and exocytosis as well as the incorporation of transmembrane proteins into cell membranes. A variety of synthetic approaches have been explored by scientists in order to accomplish such control in manmade systems. Examples include hybrid systems whereby transmembrane proteins were incorporated as part of synthetic vesicles, and the use of responsive macromolecular building blocks to regulate membrane porosity upon an external trigger in polymer vesicles, also referred to as polymersomes.
Dark field microscopy image of polymer vesicles which contain manganese oxide colloidal particles in their membranes, pre-loaded with barium ions and dispersed in water (containing sulfate anions) after their exposure to a low external concentration of hydrogen peroxide. The white haze surrounding the vesicles and the white tails are the result of the precipitation of barium sulfate crystals upon release of the barium cations from the vesicles. Scale bar: 100 μm
In our recent paper published in Materials Horizons we show for the first time that the permeability of the membrane of polymer vesicles can be controlled by membrane-embedded catalytically active manganese oxide particles. The ability to chemically trigger activity of the catalytic particle, hereby inducing a temporary increase of membrane permeability, offers precise time-specific control of transmembrane transport. It is our belief that this concept can be applied to a wide variety of membrane-based systems. At the end of the paper we open a disccusion for the origins of life and protocell communities that a hybrid vesicular structure, which has “active” colloidal particles as part of its membrane, may have regulated permeability in primitive cells.
to read the paper: http://dx.doi.org/10.1039/C5MH00093A
A Mechanistic Insight into the Synthesis of Silica-Based “Matchstick” Colloids
In the field of colloid science the ability to fabricate particles with a defined shape, other than a sphere, has gained attention. The reason is that anisotropy in shape and/or chemical composition can lead to interesting physical properties when these particles are dispersed in a liquid, or when they form part of a product formulation. We report an insight into the synthesis of silica-based “matchstick”-shaped colloidal particles, which are of interest in the area of self-propulsion on small length scales. The generation of aqueous emulsion droplets dispersed in an n-pentanol-rich continuous phase and their use as reaction centers allows for the fabrication of siliceous microparticles that exhibit anisotropy in both particle morphology, that is, a “matchstick” shape, and chemistry, that is, a transition-metal oxide-enriched head. We provide a series of kinetic studies to gain a mechanistic understanding and unravel the particle formation and growth processes. Additionally, we demonstrate the ability to select the aspect ratio of the “matchstick” particle in a straightforward manner.
The paper is recently published in Langmuir. DOI:10.1021/acs.langmuir.5b02645
In the field of colloid science the ability to fabricate particles with a defined shape, other than a sphere, has gained attention. The reason is that anisotropy in shape and/or chemical composition can lead to interesting physical properties when these particles are dispersed in a liquid, or when they form part of a product formulation. We report an insight into the synthesis of silica-based “matchstick”-shaped colloidal particles, which are of interest in the area of self-propulsion on small length scales. The generation of aqueous emulsion droplets dispersed in an n-pentanol-rich continuous phase and their use as reaction centers allows for the fabrication of siliceous microparticles that exhibit anisotropy in both particle morphology, that is, a “matchstick” shape, and chemistry, that is, a transition-metal oxide-enriched head. We provide a series of kinetic studies to gain a mechanistic understanding and unravel the particle formation and growth processes. Additionally, we demonstrate the ability to select the aspect ratio of the “matchstick” particle in a straightforward manner.
The paper is recently published in Langmuir. DOI:10.1021/acs.langmuir.5b02645
Fabrication of calcium phosphate microcapsules using emulsion droplets stabilized with branched copolymers as templates
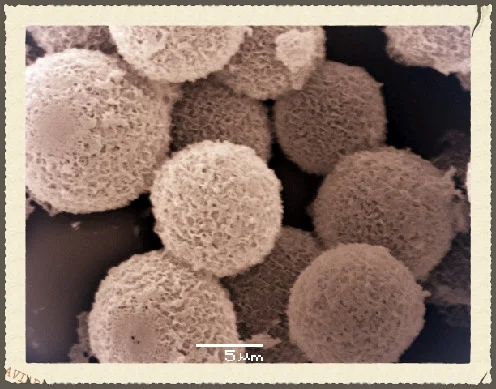
Calcium phosphate based hybrid materials are of great interest for bio-related science, for example our bones and teeth contain mineral components made from calcium phosphate. One class of materials of great interest are microcapsules, as these can store and release active ingredients. Calcium phosphate microcapsules have been made before via a number of synthetic pathways. Key drawbacks however are tedious and long (up to a month) fabrication methods. In our paper published recently in the Journal of Materials Chemistry B we report on a versatile and time-efficient method to fabricate calcium phosphate (CaP) microcapsules by utilizing oil-in-water emulsion droplets stabilized with synthetic branched copolymer (BCP) as templates. The BCP was designed to provide a suitable architecture and functionality to produce stable emulsion droplets, and to permit the mineralization of CaP at the surface of the oil droplet when incubated in a solution containing calcium and phosphate ions. The CaP shells of the microcapsules were established to be calcium deficient hydroxyapatite with incorporated chlorine and carbonate species. These capsule walls were made fluorescent by decoration with a fluorescein-bisphosphonate conjugate.
To read the paper: DOI: 10.1039/C5TB00893J
SEM micrographs illustrating the mineralization of CaP at the surface of oil droplets stabilized with BCP. (A) Incubation periods of 0 hours (scale bar = 37 µm), (B) 48 hours (scale bar = 16 µm), (C) 60 hours (scale bar = 7 µm), (D and E) 72 hours (scale bars = 23 µm and 7 µm, respectively), (F) surface morphology of CaP capsule (scale bar = 704 nm), (G) CaP capsules annealed at 600 oC (scale bar = 2 µm), (H) surface morphology of CaP capsule after annealing at 600 oC (scale bar = 648 nm), and (I and J) shell thickness of the CaP capsules before annealing (scale bars = 1 µm and 540 nm, respectively).
Calcium phosphate based hybrid materials are of great interest for bio-related science, for example our bones and teeth contain mineral components made from calcium phosphate. One class of materials of great interest are microcapsules, as these can store and release active ingredients. Calcium phosphate microcapsules have been made before via a number of synthetic pathways. Key drawbacks however are tedious and long (up to a month) fabrication methods. In our paper published recently in the Journal of Materials Chemistry B we report on a versatile and time-efficient method to fabricate calcium phosphate (CaP) microcapsules by utilizing oil-in-water emulsion droplets stabilized with synthetic branched copolymer (BCP) as templates. The BCP was designed to provide a suitable architecture and functionality to produce stable emulsion droplets, and to permit the mineralization of CaP at the surface of the oil droplet when incubated in a solution containing calcium and phosphate ions. The CaP shells of the microcapsules were established to be calcium deficient hydroxyapatite with incorporated chlorine and carbonate species. These capsule walls were made fluorescent by decoration with a fluorescein-bisphosphonate conjugate.
To read the paper: DOI: 10.1039/C5TB00893J