BONLAB BLOG
Thoughts
&
Scientific Fiction
BonLab joins the Bio Electricity Group and the Bio Electrical Engineering (BEE) Hub
The BonLab at Warwick University specialises in the fabrication of colloidal and macromolecular materials for a wide range of applications, including coatings/adhesives, personal/household care products, and confectionary. BonLab's recent scientific activity in the fields of autonomous and programmable colloidal gels and active colloidal matter drew attention from researchers in life sciences.
Prof.dr.ir. Stefan Bon says: "We are delighted with the invitation to join the bio electricity group and the bio electrical engineering (BEE) hub, hosted at Warwick University. We hope that our scientific portfolio and know-how will provide a synergistic angle and will help innovate in this exciting area of science"
Information on the Bio Electricity Group:
Despite the early works of Luigi Galvani in the 18th century, the experimental inquiry into the biological systems has never fully taken an electrical viewpoint. Galvani’s, and subsequently Alessandro Volta’s, studies led to the discovery of the electrical battery and the birth of electrochemistry, but the biological thread have been largely neglected outside of neurosciences.
At Warwick, we have taken on this neglected thread and have identified biological electricity as a key research direction. In particular, we believe that electrical forces, and the ability to control them, are fundamental in organising living systems across the scales (see publications). To better understand these forces and develop means to measure and control them, we undertake an interdisciplinary approach that brings together expertise from biology, physics, engineering, and chemistry.
Our research in this area is currently conducted through several collaborative PhD and postdoctoral projects. In addition, we have recently launched a Bio Electrical Engineering (BEE) Innovation Hub with funds from a BBSRC Innovation Accelarator Award provided to the University of Warwick.
Current membership (and interest areas) in the Warwick BioElectricity group include; Munehiro Asally (electrical patterns in cellular organisation), Orkun Soyer (electrical interfaces to cells), Murray Grant (electrical signals in plants), Pat Unwin (electrobiochemical measurements), Marco Polin (electrotaxis), Rob Cross (sub-cellular electrical fields), and Stefan Bon (electrical stimuli in colloidal biomaterials)
.
Assembly of colloidal latex particles leads to innovation in fabrication of porous materials
Porous materials that have an interconnected network of pores are an interesting class of materials and have drawn attention in the area of separation science. The ability to fabricate robust so-called open cellular materials with control of the porosity remains a scientific challenge. The ability of regulating the interconnected network determines how a fluid (liquid or gas) can flow through the system. Think for example of how water runs through soil, or how water can be taken up through capillary action into a sponge. In addition, one can foresee that matter which flows through the porous material can temporarily be adhered/adsorbed onto the surface of the porous monolithic structure. The ability to easily control the surface functionality of the walls of the pores therefore is important.
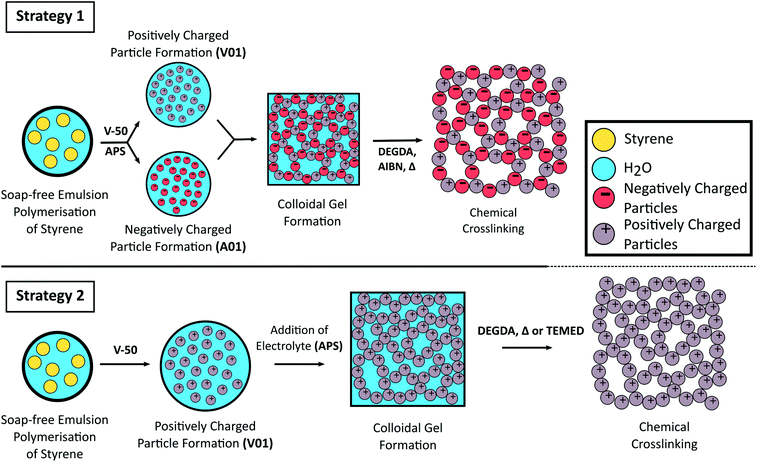
In collaborative work with Chris Desire, a talented PhD student from the group of prof. Emily Hilder at the University of South Australia, we in the BonLab describe in Green Chemistry that we can use polymer latex particles as colloidal building blocks to form robust open cellular porous monolithic materials by simply stacking them onto each other. This assembly process is triggered by colloidal instability of a polymer latex dispersed in water which leads to the formation of a colloidal gel. The structure of the gel can then be made permanent by cross-linking through polymerization.
Porous materials that have an interconnected network of pores are an interesting class of materials and have drawn attention in the area of separation science. The ability to fabricate robust so-called open cellular materials with control of the porosity remains a scientific challenge. The ability of regulating the interconnected network determines how a fluid (liquid or gas) can flow through the system. Think for example of how water runs through soil, or how water can be taken up through capillary action into a sponge. In addition, one can foresee that matter which flows through the porous material can temporarily be adhered/adsorbed onto the surface of the porous monolithic structure. The ability to easily control the surface functionality of the walls of the pores therefore is important.
In collaborative work with Chris Desire, a talented PhD student from the group of prof. Emily Hilder at the University of South Australia, we in the BonLab describe in Green Chemistry that we can use polymer latex particles as colloidal building blocks to form robust open cellular porous monolithic materials by simply stacking them onto each other. This assembly process is triggered by colloidal instability of a polymer latex dispersed in water which leads to the formation of a colloidal gel. The structure of the gel can then be made permanent by cross-linking through polymerization.
Schematic representation for the formation of crosslinked colloidal gels from oppositely charged latex particles prepared from the soap-free emulsion polymerisation of styrene using different initiators (Strategy 1) or from the addition of electrolyte to a cationic polymer latex (Strategy 2).
The pore size of the resulting monoliths was predictable as this was observed to directly correlate to the particle diameter, with larger pores achieved using particles of increased size. All gels obtained in this work were highly mouldable and retained their shape, which allowed for a range of formats to be easily prepared without the requirement of a mould.
Our innovation is applicable for the preparation of polymer monoliths for a wide variety of applications, including but not limited to, tissue engineering, catalysis, chromatography, extraction, sample preparation, and as absorbents. In particular these monoliths were found to posses relatively high porosities and were capable of rapidly absorbing solvents of varying polarity by capillary action, which suggested their applicability for thin layer chromatography (diagnostics) and extraction.
The original paper is published in Green Chemistry DOI:10.1039/C8GC01055B
BonLab does PISA with RAFT-agent version 0.1
Call them plastics, polymers, elastomers, thermoplasts, thermosets, or macromolecules. What’s in the name? Despite the current negative press in view of considerable environmental concerns on how we deal with polymer materials post-use, it cannot be denied that polymers have been a catalyst in the evolution of human society in the 20st century, and continue to do so.
One of the synthetic pathways toward polymer molecules is free radical polymerization, a process known since the late 1800s and conceptually developed from the 1920s-1930s onwards. Since the 1980s it gradually became possible to tailor the chemical composition and chain architecture of a macromolecule. The process is called reversible deactivation radical polymerization (RDRP), also known as controlled or living radical polymerization. By grabbing control on how individual polymer chains are made, with the ability to control the sequencing of its building blocks, known as monomers, true man-made design of large functional molecules has become reality. This architectural control of polymer molecules allows for materials to be formulated with unprecedented physical and mechanical properties.
One interesting phenomenon is that when we carry out an RDRP reaction using a “living” polymer (a first block) dissolved in for example water and try to extend the macromolecule by growing a second block that does not dissolve in water, it is possible to arrange the blockcopolymer molecules by grouping them together into a variety of small (colloidal) structures dispersed in water. More interestingly, these assembled suprastructures have the ability to dynamically change shape throughout the polymerization process, for example to transform from spherical, to cylindrical, to vesicle type objects. This Polymerization Induced Self-Assembly process has been given the acronym PISA.
Call them plastics, polymers, elastomers, thermoplasts, thermosets, or macromolecules. What’s in the name? Despite the current negative press in view of considerable environmental concerns on how we deal with polymer materials post-use, it cannot be denied that polymers have been a catalyst in the evolution of human society in the 20st century, and continue to do so.
One of the synthetic pathways toward polymer molecules is free radical polymerization, a process known since the late 1800s and conceptually developed from the 1920s-1930s onwards. Since the 1980s it gradually became possible to tailor the chemical composition and chain architecture of a macromolecule. The process is called reversible deactivation radical polymerization (RDRP), also known as controlled or living radical polymerization. By grabbing control on how individual polymer chains are made, with the ability to control the sequencing of its building blocks, known as monomers, true man-made design of large functional molecules has become reality. This architectural control of polymer molecules allows for materials to be formulated with unprecedented physical and mechanical properties.
One interesting phenomenon is that when we carry out an RDRP reaction using a “living” polymer (a first block) dissolved in for example water and try to extend the macromolecule by growing a second block that does not dissolve in water, it is possible to arrange the blockcopolymer molecules by grouping them together into a variety of small (colloidal) structures dispersed in water. More interestingly, these assembled suprastructures have the ability to dynamically change shape throughout the polymerization process, for example to transform from spherical, to cylindrical, to vesicle type objects. This Polymerization Induced Self-Assembly process has been given the acronym PISA.
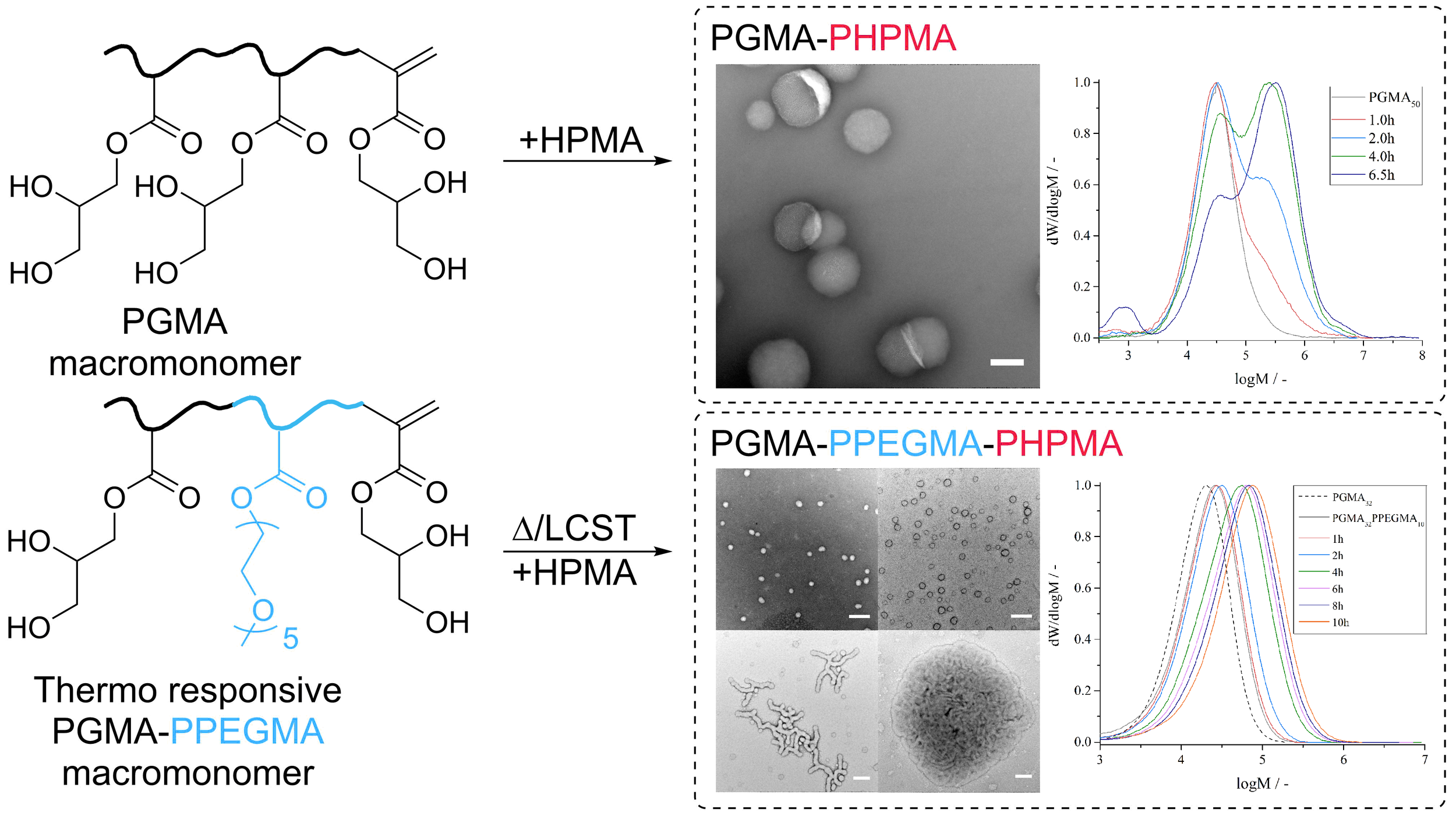
One way to carry out the synthesis of macromolecules by reversible deactivation radical polymerization is to make use of the concept of Reversible Addition-Fragmentation chain-Transfer, known as RAFT. This polymerization process has conventionally now uses sulfur-chemistry in the synthesis of RAFT-agents (versions 1.0 and up so to say), which efficiently controlled the growth of macromolecules. It is based on an invention from the mid 1990s, but more interestingly came to fruition by the realization that methacrylate-based macromonomers acted as RAFT-agents (here version 0.1). These latter compounds were, however, not very efficient, and abandoned.
We now show in the BonLab’s latest paper published in ACS Macro Letters that methacrylate-based macromonomers can be used successfully as RAFT-agents in Polymerization Induced Self-Assembly (PISA) processes by carefully considering the mechanistic aspects.
Prof.dr.ir. Stefan Bon says: “Our team is delighted with the results and we are happy the paper features in ACS Macro Letters. RAFT-ing the classic way will for sure get more than a 2nd try! To show that PISA with version 0.1 RAFT-agents is indeed possible, was an excellent team effort by PhD researcher Andrea Lotierzo, and 2nd year undergraduate Warwick Chemistry student (now 3rd year) Ryan Schofield.”
The use of this class of "primitive" RAFT-agents in heterogeneous polymerizations is however not trivial, because of their inherent low reactivity. In our work we demonstrate that two obstacles need to be overcome, one being control of chain-growth (propagation), the other monomer partitioning. Batch dispersion polymerizations of hydroxypropyl methacrylate in presence of poly(glycerol methacrylate) macromonomers in water showed limited control of chain-growth. Semi-continuous experiments whereby monomer was fed improved results only to some extent. Control of propagation is essential for PISA to allow for dynamic rearrangement of colloidal structures. We tackled the problem of monomer partitioning (caused by uncontrolled particle nucleation) by starting the polymerization with an amphiphilic thermo-responsive diblock copolymer, already “phase-separated” from solution. TEM analysis showed that PISA was successful and that different consecutive particle morphologies were obtained throughout the polymerization process.
The paper can be downloaded from ACS Macro Letters. DOI: 10.1021/acsmacrolett.7b00857