BONLAB BLOG
Thoughts
&
Scientific Fiction
Structure and behaviour of vesicles in presence of colloidal particles
We in the BonLab have occasionally played with vesicles, microscopic sacs dispersed in a liquid medium that enclose finite volumes of the liquid, thereby compartmentalising it from the bulk phase by a thin membrane.
In our 2011 JACS paper we showed that we could decorate polymer vesicles with a layer of colloidal particles, effectively creating an armour. These colloidal particles could be silica nanoparticles, or polymer latex particles. The latter even had the ability to film-form, or to transform into a hydrogel. When monodisperse latexes were used the 2D layer organised itself as a colloidal crystal.
In our 2016 Materials Horizons cover paper we showed that we spatially and temporally could regulate the membrane permeability of vesicular structures by embedding catalytic colloidal particles in a trans-membrane fashion.
Fascinating work has been done in this area by many others. We thought it was a nice idea to highlight these discoveries and bring them together in a review. It features recent studies that investigate the structural changes and behaviour of synthetic vesicles when they are exposed to colloidal particles. We will show examples to demonstrate the power of combining particles and vesicles in generating exciting supracolloidal structures. These suprastructures have a wide range of often responsive behaviours that take advantage of both the mechanical and morphological support provided by the vesicles and the associated particles with preset functionality. Since our review includes applications spanning a variety of disciplines, including chemistry, biology, physics and medicine, we felt that Soft Matter was the right place to publish.
We hope you enjoy reading it.
To read the review: DOI: 10.1039/C8SM01223G
BonLab joins the Bio Electricity Group and the Bio Electrical Engineering (BEE) Hub
The BonLab at Warwick University specialises in the fabrication of colloidal and macromolecular materials for a wide range of applications, including coatings/adhesives, personal/household care products, and confectionary. BonLab's recent scientific activity in the fields of autonomous and programmable colloidal gels and active colloidal matter drew attention from researchers in life sciences.
Prof.dr.ir. Stefan Bon says: "We are delighted with the invitation to join the bio electricity group and the bio electrical engineering (BEE) hub, hosted at Warwick University. We hope that our scientific portfolio and know-how will provide a synergistic angle and will help innovate in this exciting area of science"
Information on the Bio Electricity Group:
Despite the early works of Luigi Galvani in the 18th century, the experimental inquiry into the biological systems has never fully taken an electrical viewpoint. Galvani’s, and subsequently Alessandro Volta’s, studies led to the discovery of the electrical battery and the birth of electrochemistry, but the biological thread have been largely neglected outside of neurosciences.
At Warwick, we have taken on this neglected thread and have identified biological electricity as a key research direction. In particular, we believe that electrical forces, and the ability to control them, are fundamental in organising living systems across the scales (see publications). To better understand these forces and develop means to measure and control them, we undertake an interdisciplinary approach that brings together expertise from biology, physics, engineering, and chemistry.
Our research in this area is currently conducted through several collaborative PhD and postdoctoral projects. In addition, we have recently launched a Bio Electrical Engineering (BEE) Innovation Hub with funds from a BBSRC Innovation Accelarator Award provided to the University of Warwick.
Current membership (and interest areas) in the Warwick BioElectricity group include; Munehiro Asally (electrical patterns in cellular organisation), Orkun Soyer (electrical interfaces to cells), Murray Grant (electrical signals in plants), Pat Unwin (electrobiochemical measurements), Marco Polin (electrotaxis), Rob Cross (sub-cellular electrical fields), and Stefan Bon (electrical stimuli in colloidal biomaterials)
.
Assembly of colloidal latex particles leads to innovation in fabrication of porous materials
Porous materials that have an interconnected network of pores are an interesting class of materials and have drawn attention in the area of separation science. The ability to fabricate robust so-called open cellular materials with control of the porosity remains a scientific challenge. The ability of regulating the interconnected network determines how a fluid (liquid or gas) can flow through the system. Think for example of how water runs through soil, or how water can be taken up through capillary action into a sponge. In addition, one can foresee that matter which flows through the porous material can temporarily be adhered/adsorbed onto the surface of the porous monolithic structure. The ability to easily control the surface functionality of the walls of the pores therefore is important.
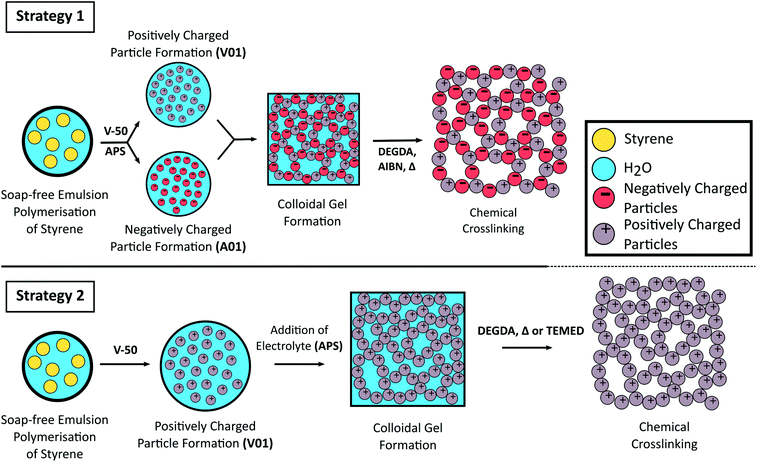
In collaborative work with Chris Desire, a talented PhD student from the group of prof. Emily Hilder at the University of South Australia, we in the BonLab describe in Green Chemistry that we can use polymer latex particles as colloidal building blocks to form robust open cellular porous monolithic materials by simply stacking them onto each other. This assembly process is triggered by colloidal instability of a polymer latex dispersed in water which leads to the formation of a colloidal gel. The structure of the gel can then be made permanent by cross-linking through polymerization.
Porous materials that have an interconnected network of pores are an interesting class of materials and have drawn attention in the area of separation science. The ability to fabricate robust so-called open cellular materials with control of the porosity remains a scientific challenge. The ability of regulating the interconnected network determines how a fluid (liquid or gas) can flow through the system. Think for example of how water runs through soil, or how water can be taken up through capillary action into a sponge. In addition, one can foresee that matter which flows through the porous material can temporarily be adhered/adsorbed onto the surface of the porous monolithic structure. The ability to easily control the surface functionality of the walls of the pores therefore is important.
In collaborative work with Chris Desire, a talented PhD student from the group of prof. Emily Hilder at the University of South Australia, we in the BonLab describe in Green Chemistry that we can use polymer latex particles as colloidal building blocks to form robust open cellular porous monolithic materials by simply stacking them onto each other. This assembly process is triggered by colloidal instability of a polymer latex dispersed in water which leads to the formation of a colloidal gel. The structure of the gel can then be made permanent by cross-linking through polymerization.
Schematic representation for the formation of crosslinked colloidal gels from oppositely charged latex particles prepared from the soap-free emulsion polymerisation of styrene using different initiators (Strategy 1) or from the addition of electrolyte to a cationic polymer latex (Strategy 2).
The pore size of the resulting monoliths was predictable as this was observed to directly correlate to the particle diameter, with larger pores achieved using particles of increased size. All gels obtained in this work were highly mouldable and retained their shape, which allowed for a range of formats to be easily prepared without the requirement of a mould.
Our innovation is applicable for the preparation of polymer monoliths for a wide variety of applications, including but not limited to, tissue engineering, catalysis, chromatography, extraction, sample preparation, and as absorbents. In particular these monoliths were found to posses relatively high porosities and were capable of rapidly absorbing solvents of varying polarity by capillary action, which suggested their applicability for thin layer chromatography (diagnostics) and extraction.
The original paper is published in Green Chemistry DOI:10.1039/C8GC01055B