BONLAB BLOG
Thoughts
&
Scientific Fiction
Fibers made by assembly of emulsion droplets
Fibers are interesting. They are made by a spinning process in which a liquid based mixture, referred to as spinning dope, is extruded through an orifice hereby generating a jet, which subsequently is solidified through either coagulation/precipitation and/or gelation. Two extreme fibers found in Nature are spidersilk, a super strong and extensible liquid-crystalline fiber, and the soft hydrogel double-strings of toad eggs, as spawn by the common toad (Bufo bufo). The production of manmade fibers using dry and wet spinning techniques – both starting from a liquid mixture – goes back to the 19th century. An early example is the development of Rayon fibers initiated by the discovery of Schweizer in 1857, who found that cellulose could be dissolved in and re-precipitated from an aqueous solution of ammonia and copper (II) hydroxide (coined Schweizer’s reagent (dry or wet)). Examples of wet-spun high performance fibers include ultrahigh molecular weight poly(ethylene) fibers, and polyaramid fibers.
An emerging trend is to make soft, hydrogel-based, fibers wet spun into water. Applications for example are in the area of tissue engineering. Microfluidic technologies are often employed to manufacture these fibers.
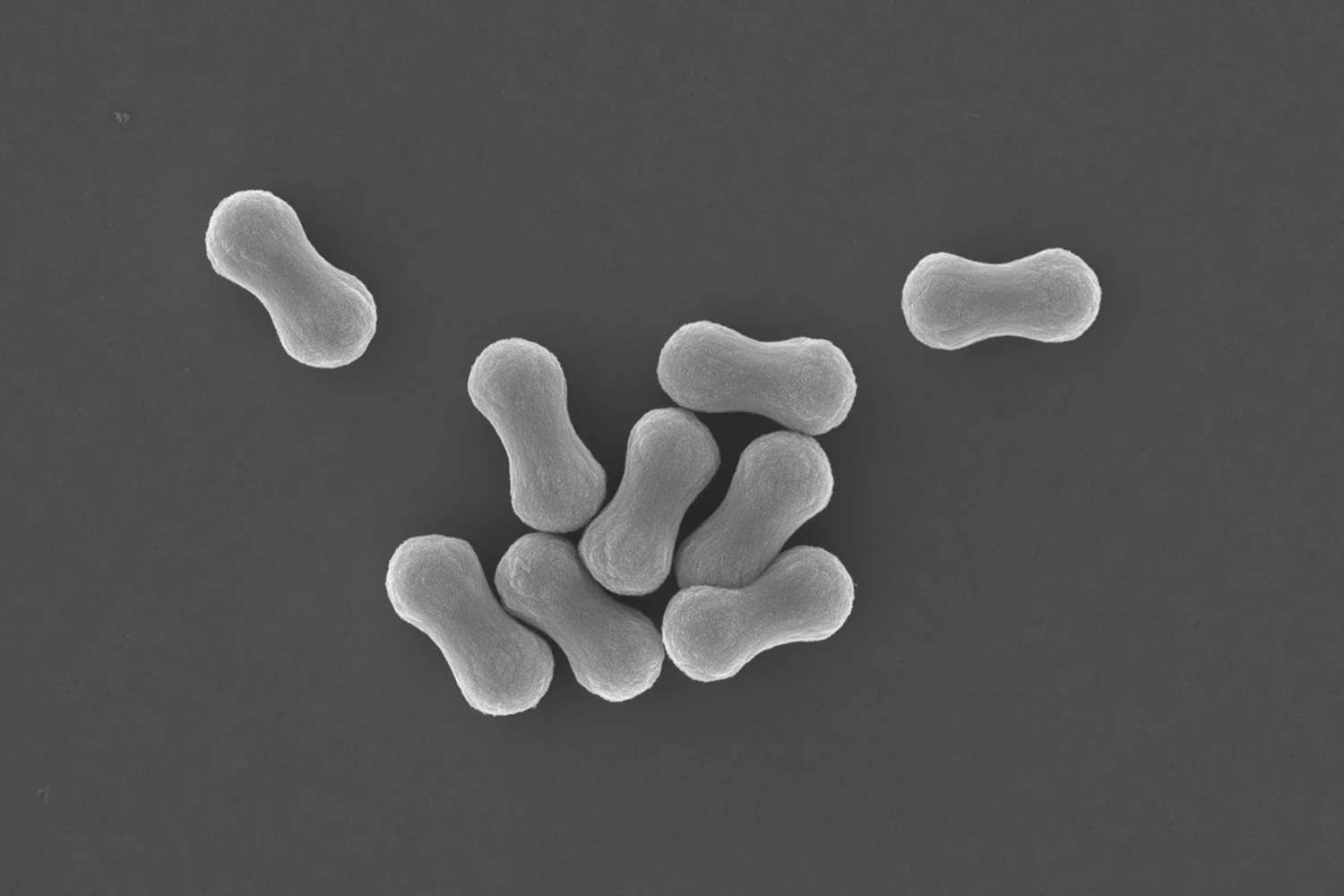
We asked ourselves whether it would be possible to fabricate fibers through assembly of thousands of emulsion droplets? We call these HIPE (High Internal Phase Emulsion) fibers.
Fibers are interesting. They are made by a spinning process in which a liquid based mixture, referred to as spinning dope, is extruded through an orifice hereby generating a jet, which subsequently is solidified through either coagulation/precipitation and/or gelation. Two extreme fibers found in Nature are spidersilk, a super strong and extensible liquid-crystalline fiber, and the soft hydrogel double-strings of toad eggs, as spawn by the common toad (Bufo bufo). The production of manmade fibers using dry and wet spinning techniques – both starting from a liquid mixture – goes back to the 19th century. An early example is the development of Rayon fibers initiated by the discovery of Schweizer in 1857, who found that cellulose could be dissolved in and re-precipitated from an aqueous solution of ammonia and copper (II) hydroxide (coined Schweizer’s reagent (dry or wet)). Examples of wet-spun high performance fibers include ultrahigh molecular weight poly(ethylene) fibers, and polyaramid fibers.
An emerging trend is to make soft, hydrogel-based, fibers wet spun into water. Applications for example are in the area of tissue engineering. Microfluidic technologies are often employed to manufacture these fibers.
We asked ourselves whether it would be possible to fabricate fibers through assembly of thousands of emulsion droplets? We call these HIPE (High Internal Phase Emulsion) fibers.
Fig. (a) Schematic representation of the fabrication of microfluidically spun HIPE fiber. The diagram shows coaxial flow channels in the microfluidic device, where the inner and outer capillaries contain concentrated emulsion and acidic water, respectively, in a continuous flow. A continuous HIPE fiber is produced upon exposure of the emulsion to the acidic water. The acidic conditions allow the formation of multiple hydrogen bonds between emulsion droplets, initiating them to assemble into a macroscopic supracolloidal fiber. (b) Photograph of a HIPE fiber in acidic aqueous solution (top left), and disintegration into individual dispersed emulsion droplets upon addition of base (top right). Scale bars represent 1 cm. Light micrographs of the HIPE fiber in acidic condition (bottom left) and disintegrated HIPE fiber in basic condition (bottom right). Scale bars represent 50 µm and 25 µm, respectively. (c) Photograph of HIPE fibers with uniform length. The HIPE fibers with uniform length were fabricated by utilizing air bubbles as a cutting mechanism. Scale bar represents 0.5 cm. (d) Photograph of asymmetric Janus HIPE fiber. Scale bars represent 0.1 cm (top) and 300 µm (bottom). (e) Photograph of asymmetric HIPE fiber consisting of three different sections ‘toothpaste’. Scale bars represent 0.1 cm (top) and 300 µm (bottom). (f) Photograph of magnetic HIPE fiber attracted by an external magnet (fiber with a slight yellow color - left hand side), and no magnetic response with the non-magnetic HIPE fiber (white color fiber - right hand side). Scale bar represents 1 cm.
In our paper published in the Journal of Materials Chemistry A we show the fabrication of fibers from emulsion droplets. We use flow-focussing microfluidic set-up whereby a generated jet of emulsion droplets stabilized by amphiphilic pH-responsive branched copolymers (pH-BCP) and reinforced by Laponite clay discs is exposed to an external surrounding liquid flow of lower pH. Proton diffusion into the stream of emulsion droplets induces the self-assembly process leading to the formation of a continuous supracolloidal fiber. We demonstrate that the fiber can disintegrate back into an oil-in-water emulsion. We discuss control of fiber composition hereby using two and three combined streams of emulsion droplets to generate Janus fibers, and using ferrofluids to produce magnetic fibers. We show control of fiber length by employing air bubbles as a mean to produce short fibers of discrete length. Looking towards applications we demonstrate the use of our supracolloidal emulsion droplet fibers as a material to control the delivery of volatile compounds through evaporation, and we show that the dried fibers are a nanocomposite highly porous and light material.
You can read the paper here: http://dx.doi.org/10.1039/C5TA08917D
Corinna Preuss awarded Newton International Fellowship
Dr Corinna Preuss has been awarded a Newton International Fellowship to conduct research at the University of Warwick’s Department of Chemistry.
Corinna Preuss
Jointly run by The British Academy, The Academy of Medical Sciences and the Royal Society, the Fellowship is for non-UK scientists who are at an early stage of their research career and provides the opportunity for the best early stage post-doctoral researchers from all over the world to work at UK research institutions for a period of two years.
Speaking after being awarded the Fellowship Dr Preuss said:
“I’m very honoured and delighted to be awarded the Newton International Fellowship. Not only will it support my personal development but is also emphasises the novelty and importance of our proposed research project.”
Dr Preuss will work as part of a team led by Professor Stefan Bon to mimic the motional behaviour of zooplankton by fabricating artificial jelly-objects that have the capability to transform shape, swim, and – as an additional feature – release payloads. Dr Preuss says these hydrogel objects will have these three pre-programmed functions “which can be triggered on demand in a controlled fashion”. For this purpose, recent scientific advances in polymer and colloid chemistry will be merged with soft matter physics and robotics in order to create a promising and interdisciplinary research program
Further to the research with Professor Bon, Dr Preuss is keen to use the Fellowship to teach undergraduate chemists and to create a network with other fellow scientists, saying that: “In my opinion, exchanging knowledge and listening to different opinions is essential for the formation of a highly efficient scientific society”.
Discussing why she chose the University of Warwick Dr Preuss said:
“I met Professor Stefan Bon during a conference in Mexico.I was impressed by his research and the passion he presented it with. Later on, whilst I was presenting my research at the poster session, we got the chance to chat more and discovered that our interests in each other’s research would create a promising base for a further collaboration. In working with Stefan and coming to the University of Warwick, I’m taking the chance of changing my field of research to colloidal chemistry and engineering, which provides a new, challenging and fascinating area for me”.
Contacts:
Tom Frew - International Press Officer
Email: a.t.frew@warwick.ac.uk
Tel: +44 (0)247 657 5910
Mob: +44 (0)7785 433 155
Ross Jaggers invited to participate in the Stonewall Young Leaders Programme
Ross Jaggers, a second year PhD student in the research group of prof.dr.ir. Stefan Bon (BonLab) in the Department of Chemistry at the University of Warwick, has been invited to attend the Stonewall Young Leaders Programme in London this coming September. The programme, sponsored by Bank of America Merrill Lynch, explores how sexual orientation and gender identity relates to the workplace and career aspirations, as well as inspiring participants to think about their impact on others as young LGBT leaders and role models.
Ross Jaggers
For more information on Stonewall, the UK’s leading LGBT rights charity, visit www.stonewall.org.uk.







